Neovascularisation : What is the surgeon’s responsability ?

Neovascularisation : What is the surgeon’s responsability ?
RESUME
Plusieurs causes induisent la néovascularisation: facteur de croissance endothélial vasculaire, inflammation, hypertrophie de vaisseaux pré-existant (veine lymphoganglionnaire), revascularisation d’hématomes et différence de pression. Les 3 dernières dépendent directement de la façon dont est réalisée l’opération, donc du chirurgien.
Concernant les varices primaires l’interposition d’un patch ne supprime pas la néovascularisation. Il est très important de ne faire une crossectomie que lorsqu’il y a une incontinence des valves terminales et préterminales. Dans ce cas l’incision doit être la plus petite possible et réalisée avec une dissection minimum, avec une tumescence afin de réduire le saignement et les hématomes. Quand la valve terminale est continente il est préférable d’utiliser une technique endovasculaire.
Concernant la récidive il est important d’éviter les reprises chirurgicales inutiles, le meilleur choix est la sclérothérapie peropératoire. Je suis convaincu que la néovascularisation est produite par les complications, elle-même induites par les dissections extensives. L’interposition d’un patch n’est certainement pas aussi nécessaire que nous l’avons prétendu 10 ans auparavant. Eviter tout traumatisme pendant l’intervention est certainement beaucoup plus important : « faire moins au niveau de l’aine pour faire mieux dans le risque de récidive et de re-récidive »
SUMMARY
There are different causes of neovascularisation : vascular endothelial growth factor, inflammatory process, hypertrophy of pre-existant vessels (lymph node veins), haematomas revascularisation, and high difference in pressure. The latter 3 depend directly on the way the operation is performed hence on the surgeon.
Concerning the primary varices patch saphenoplasty do not abolish neovascularisation. It is of first importance to perform a flush ligation only in case of incontinent terminal and pre-terminal valves. In that case an incision as small as possible must be carried out with minimal dissection and with tumescence to limit the bleeding and hematomas. When the terminal valve is competent it is better to choose endovascular technique.
Concerning re-do it is mandatory to avoid useless re-do, the best choice is foam sclerotherapy. I am convinced that neovascularisation are produced by the complications which are induced by a large dissection. The barrier technique are, probably, not as useful as we was told 10 years ago. The lack of aggressiveness during the operation is certainly far more important: “Doing less in the groin to do better for the recurrence and re-recurrence”
In the context of varicose recurrence after great saphenous vein (GSV) surgery, the term neovascularisation describes the phenomenon of formation of new venous channels between the saphenous stump on the common femoral vein (CFV) and the residual GSV or its tributaries.
Despite the fact that this frustrating phenomenon is frequently encountered by each vascular surgeon, even by those well trained, after apparently having performed a correct and complete operation its nature and pathophysiology (hence its prevention) are poorly understood and the subjects of intensive controversial discussion [1, 2]. Neovascularisation is frustrating for a good well trained and skilled vascular surgeon, although surgeon is sometimes responsible for the development of neovascularisation. The surgeon is not always an “innocent bystander”
The problem is to answer these 2 questions: how and why does neovascularisation happen and how can this development be avoided or limited ?
DEFINITION
In 1861, Langenbeck [3] described for the first time in detail the formation of new veins after ligation. In his article we can read a description of neovascularisation: “ln one case of very large varix of the great saphena in a young man I had extirpated the enlarged vein in the length of three inches and ligated the upper and lower ends. One year later I found, in the region of the scar tissue of the extirpation, a new vein channel of the thickness of the quill of a crow’s feather, which again joined the both ends of the fully functioning saphena”
Twenty years ago a description was done by Glass in 1987 [4]. In his work a series of patients with venous ulceration due to GSV insufficiency were treated in stages. First, a transection of GSV was performed in the lower part of the thigh. In a second stage, individually timed for each patient by healing of the ulcer, the sapheno femoral junction (SFJ) was ligated in combination with stripping of the trunk. During the same operation the ends of the vein at the site of the original transection in the lower thigh were excised together with the tissue intervening between them. At 6 week follow-up (FU) he could see a recanalisation of the thrombus, at 18 week new vessels and at 40 week the continuity was completely restored. He hypothesized that one of the important triggers for restoration of continuity was a large pressure difference in the proximal and distal parts of the vein.
CAUSES OF NEOVASCULARISATION
First of all, the physiopathology of neovascularisation seems to have something in common with the physiopathology of the varicose veins [5]. The physiopathology of varicose veins involves genetic mechanisms, reduced apoptosis, increasing in collagen and decrease in elastin, decreased endothelin β receptor, vascular endothelial growth factor (VEGF) and inflammatory process. Only the 2 last one are concerned in the neovascularisation.
VEGF
It is known that plasma levels of VEGF are higher in chronic venous disease (CVD) patients than in normal controls [6]. Such stump-related neovascularisation might originate from hypoxia-induced activation of endothelial cells distal to the stump ligature, which could be mediated by growth factors [7]. VEGF are the strongest stimulators for angiogenesis. In a study of Rewerk [8] patients were distributed into a control group, a primary varicose vein group and a recurrent varicose vein group. Thin-walled veins embedded in scar tissue (neovascularisation) were stained immunohistochemically with antibodies against VEGF. Presence and role of VEGF was demonstrated by a higher staining intensity seen in the recurrence group.
Inflammatory process
Strong evidence has now been gathered to indicate that chronic venous insufficiency (CVI) is an inflammatory disease. Actually, ultrastructural and immunohistochemical studies of valves and the venous wall have revealed leukocytes adhering and transmigrating into the venous wall. The suggestion that inflammation may be involved in CVD comes from the evidence for elevated endothelial permeability [9]. Inflammation that is involved in postoperative cicatrisation is also involved in CVD. An analysis may lead us toward the trigger mechanisms for inflammation in CVD. An inflammatory process may be involved particularly at the cellular and molecular levels and may be the origin of the earliest manifestations of varicose veins
Role of the venous pressure in neovascularisation development
The venous pressure could be an inductor of neovascularisation by the way of difference in pressure, variation in pressure, irregular displacement of blood and specially by the fluid shear stress which is the tangential force induced by moving blood acting on the endothelial surface and a function of the velocity gradient of blood near the endothelial surface [10].
The difference in pressure already induces vascular remodelling as demonstrated by El Wajeh [11]. The venous channels that develop at the previously ligated SFJ may represent adaptive dilatation of pre-existing venous channels (vascular remodelling), probably in response to abnormal haemodynamic forces. Tissue samples from the region of the previously ligated SFJ were taken from 14 limbs with recurrent varicose veins and 9 control limbs. Tissue samples were analysed histologically, and immunohistoligically for S100, a neural marker, and Ki-67 (Mib1), a marker of endothelial proliferation. No significant difference was found between the venous recurrence and control groups in respect to histological features.
The difference in pressure induces also inflammation as described by Takase [12] who demonstrated that short-term venous pressure elevations caused elevated microvascular permeability (blue escape across the endothelium was evidence for elevation of venular permeability and extravasation of red cells across postcapillary venules was evidence of inflammation)
Shear stress could be an inductor of neovascularisation. Owatverot [13] demonstrated that low flow or flow disturbances (that is typically located in the groin area), especially if they involve instances of reversed flow direction with forward and backward shear, make cells more susceptible to inflammatory mediators. Stretching of endothelial cells and smooth muscle cells also has a direct effect on many aspects of the endothelial biology including synthesis and release of many inflammatory molecules such as leukotrienes, prostaglandin, bradykinin, free oxygen radical, and cytokines. It is typically what happens in the area of the groin.
Shear stress increases the inflammatory activity. Many authors demonstrated that both VEGF expression and its receptor expression are up-regulated under variations of blood shear stress and with the inflammatory reaction [14-16]
Role of pre-existant vessels : lymph node veins
The hypertrophy of pre-existing vessels which provide a connection between the femoral vein, the abdominal sub cutaneous vein, pelvic veins, inguinal veins and the residual saphenous trunk is obvious. These developments are a part of the neovascularisation [17].
Revascularisation of hematomas
Several recent studies emphasized the role of the hematomas.
The first study concerned 70 strippings [18]. At 1 week 54 legs presented with small haematomas and 16 legs with large haematomas. At 1 year only the 16 who presented previously with large hematomas presented with revascularisations. Among these, 12 had a partial revascularisation (6 distal third, 5 distal half, 1 almost complete) and 4 a complete revascularisation shown as multiple channels, in continuity with the distal segment of native SV and with reflux.
The second study concerned 102 cases [19]. At 2 month-FU, 6 patients had low velocity and long duration reflux in the haematomas in the saphenous tract which was seen to fill from either a thigh perforator or tributary. At 1 year-FU this haematoma tract had extended proximally towards the groin in 3 patients, 2 having connected to the CFV.
The aggressiveness of the operation could be an inductor of the neovascularisation by means of hematomas. In a study of Earnshaw [20], at one year-FU there were a higher rate of new neovascularisation in the group of patients operated on for re-do surgery than in the group operated on for primary varices. It is known that re-do surgery is more difficult and more aggressive than primary operation. Hematomas are more likely encountered in re-do surgery than in primary operation.
Among these different causes of neovascularisation (Table I) there are, at last, 3 which depend directly on the way the operation is performed that is to say it depends on the surgeon. Firstly there are “inflammatory cicatrisation” and “hematomas revascularisation” and secondly the “high difference in pressure” which depends on the surgical decision if for example a flush ligation alone is proposed without the ablation of the incompetent trunk which is an obvious cause of neovascularisation.
MACROSCOPIC AND MICROSCOPIC DEFINITION
Macroscopic examination [21-23] revealed several lumens in an irregular mass of vein tissue and cords or bands traversing the lumen, which suggested that the vessels were newly formed and not pre-existing. Large lymph nodes were often in close proximity to them. Neovascular channels were covered by a simple squamous endothelium overlying a medial layer consisting of two to five layers of vascular smooth muscle. They lacked elastic fibers, had no distinct intimal medial boundary, and no distinct adventitia. The histology confirmed the microscopic findings: an irregular vessel wall with a varying thickness at different points of the circumference, often with several lumens. Also typical was the presence of many small vessels close to the newly formed vessel and in neighbouring lymph nodes. A resin casting provided by van Rij [23] showed a connecting network of aboundant tortuous vessels.
Histological examination of the venous tissue blocks, excised during groin re-explorations, showed neovascularisation in 27 blocks, characterized by vein tortuosity, small size, and mural asymmetry and lack of intramural nerves on immunohistologically S 100 stained sections. Nyamekye [24] had defined 3 negative criteria: no intramural nerves, no three layered wall structure and lack of lumen regularity. Stücker [25] performing 91 histological examinations of excised venous tissue block can demonstrate the presence of neovascularisation and defined a positive criterion as scar tissue that must always surround a newly formed vein. He clearly defined the characteristics of the different elements seen in the area of neovascularisation.
- residual vein was: none of these vessels was surrounded by scar tissue. At S100 immunohistochemistry, 89% of veins stained positive for intramural nerves in the muscle layer of the media.
- neovascularisation was: oval single-or multi-channel recurrence within scar tissue, lined by an endothelial layer, a complete lack of a muscle layer, membrana elastica interna. All vessels stained negative for intramural nerves at S 100 immunohistochemistry.
- pre-existant venule hypertrophy was: asymmetric vessels without valves surrounded by plentiful fatty tissue. No scar tissue. S 100-positive intramural nerves found in the muscle.
- lymphovenous connection was: lymph node being able to obtain intrinsic lymphovenous links connecting the deep and superficiel systems, leading to nodes containing large subcapsular venous sinus and transiting veins.
To resume :
- Appearance of venous valves is evidence of a pre-existing vessel
- 3-layered complex structure of wall is an indication of a pre-existing vessel
- Surrounding scar tissue is a sign of previous surgery and neovascularisation
- Multiple channels are an indication of neovascularisation
- Multichannel recurrence surrounded by intact fatty tissue clearly refute previous surgery in the area
- Absence of S 100-positive intramural nerves is an indication of neovascularisation
WHAT CAN WE DO TO REDUCE THE RISK OF NEOVASCULARISATION ?
Primary varices
Unintentionnally the study of Nyamekye [24] gives us a part of the answer to this question. Actually, in these 28 histological analyses the 27 neovascularisations was observed only in the 27 previous flush ligations, no neovascularisation was observed in the previously untouched SFJ. The conclusion may be “to avoid neovascularisation don’t touch the SFJ”
a) The relationship between the residual stump and neovascualriwation has long been considered as evidence and historically dogma has maintained that to remove the stump would do away with the need for neovascularization, which has been the subject of numerous studies.
The concept was to obtain the elimination of the GSV stump with a vascular side clamp on the SFJ with a short venotomy that can be closed with a two-layer running suture. The incidence of recurrent reflux in the above-described technique in the long term has not yet been reported. Up to now, only preliminary results have been published. Some having had results, such a serious intervention can’t be reasonably suggested for a first cross-section.
b) Based on the idea of hiding or destroying the stump endothelium, it has been proposed to ligate the stump with a non resorbable suture and then bury the stump with a running polypropylene suture [26]. Hundred and fourteen duplex imagings at 2-year FU shown that recurrent reflux in the groin was significantly reduced from 11 to 3% by oversewing the ligated SFJ with a running polypropylene suture. But in a prospective randomized study with follow-up up to 5 years after surgery, Haas [27] could not confirm any beneficial effect of such inverting suture of the stump endothelium. On duplex ultrasound control, they still found neovascular vessels in 9% of 279 limbs operated on with this technique.
c) Other investigators have chosen to focus only on the stump endothelium, destroying it with chemical or heat cauterization, or in some instances reducing the amount of endothelium exposed by placing a second ligature near the free end of the GSV stump, all without conclusive results.
d) Spatial separation of the stump is probably the most important way to reduce the rate of neovascularisation and it is something the surgeon can easily do. Actually, it also seems important to achieve greater spatial separation between the deep veins underlying the fossa ovalis and the superficial venous system, by careful ligation of all tributaries in the groin beyond their junction and stripping the thigh incompetent portion of the GSV. The bad role of the residual incompetent trunk is proved by a study of Jones [28]. Two groups of patients were re-explored at 2-year follow-up. The 2 groups presented with quite the same rate of inguinal neovascularisation but only the group with residual incompetent trunk had clinical recurrences. The neovascularisation becomes incompetent and associated with varicose vein recurrences only when associated with an incompetent residual trunk.
Duplex and operative findings were recorded prospectively in a consecutive series of 500 limbs undergoing surgery for recurrent varicose veins [29]. Neovascularisation was identified on Duplex scanning in 41 limbs. In 27 of these, surgical exploration revealed a residual GSV stump with 1 or more significant tributaries. Each of the remaining 14 limbs had a residual incompetent thigh GSV. The conclusion was: neovascularisation is either associated with persistent reflux in the GSV stump or thigh GSV, or both. Neovascularisation alone is not a cause of recurrent varicose veins. There was no isolated neovascularisation.
e) Barrier techniques
Interposition of a physical barrier (prosthetic or anatomical) between the ligated GSV stump on the CFV and the surrounding superficial veins is a great old idea which could theoretically prevent tiny neovascular veins developing at the stump from connecting with superficial veins in the groin and thigh.
- Anatomical barrier techniques. To contain neovascularisation in the groin, the use of an anatomical barrier to cover the ligated saphenous stump has been proposed firstly by Glass [30, 31] (Facia cribriformis). While Glass’ work lacks rigor in its methodology, it has the merit of showing that the suturing of the fascia cribiformis or the interposition of a mersilene patch gave a nearly identical reduction in the percentage of neovascularization at four years: from 25% to 3% and from 25% to 1% respectively.
Interposition of an anatomical barrier by closing the cribriform fascia reduced ultrasound detected neovascularisation at the SFJ. A more complex barrier technique, described by Sheppard [32] consisted of the use of a flap of pectineus fascia at the SFJ. In a randomized controlled trial, Gibbs [33] could not confirm the previously suggested benefit of this particular and too complicated technique. - Prosthetic barrier techniques. De Maeseneer [34] compared two groups of limbs with and without a silicone implant (2 x 3 cm) and closure of the cribriform fascia. One year after the operation with duplex scanning silicone patch saphenoplasty significantly lowered the incidence of neovascularization. In this technique the saphenous stump has been ligated with a non resorbable suture and a silicone patch is fixed to the ligated stump. After the patch has been tucked under the cribriform fascia, covering in this way the anterior half of the CFV, the cribriform fascia was closed with separate stitches before closing Scarpa’s fascia. The saphenous stump was not visible anymore as it has been covered with a prosthetic and anatomical barrier. Incidence of neovascularisation on duplex examination in group without silicone patch was16.5% and in group with silicone patch 6.2% 12 months postoperatively.
Earnshaw [35] studied the results of a comparable barrier technique using a polytetrafluoroethylene (PTFE) patch (small patch, 1 x 2 cm), after one year. This study indicated that patch saphenoplasty was safe, but did not abolish neovascularisation after one year (14% re-neovascularisation in 51 primary varices and 40% re-neovascularisation in15 re-do surgery)
De Maeseneer [36] demonstrated that prosthetic or anatomical barrier techniques are equivalent techniques. In a group of 235 limbs of 193 patients an anatomical barrier was constructed by closing the cribriform fascia. Neovascularisation at the SFJ ligation site was 6.7%. This was comparable to the group of 191 limbs with silicone patch saphenoplasty (5.2%) (P=0.526).
f) Avoid the useless flush ligation.
It is of first importance to perform a flush ligation only in case of incontinent terminal and pre-terminal valves. Saphenous trunk reflux with continent SFJ represents 50% of the cases [37, 38].
When a flush ligation is necessary an incision as small as possible must be carried out with tumescence to limit the bleeding and hematomas with minimal dissection, no aggressiveness and a running suture to close the fascia cribriformis. When the terminal valve and the tributaries must be protected a section below the triburaries is performed (Photo 1) or only a section of the anterior accessory saphenous vein as well (Photo 2).
The endovascular ablation of the GS trunk with radiofrequency obliteration (RFO) (ClosurePlus®) reduced the rate of neovascularisation. In this comparative study [39], 6 of 55 (11%) legs in the open surgery group showed clear evidence of tortuous refluxing veins related to the SFJ. None of the 55 in the RFO group showed any neo-vascularisation at the SFJ (Fischer exact test P = 0.028).
Although a significant difference could not be demonstrated in the randomized EVOLVeS study [40] (endovascular ablation of the GS trunk with RFO ClosurePlus® versus stripping) at 2-year FU, 36 limbs with RFO show one neovascularisation and 29 limbs with stripping show 4 neovascularisations
Redo surgery
In the re-do surgery, because of the destruction of the cribriform fascia only prosthetic barriers are usable. Because of some particular difficulties of re-do surgery it is of the highest importance to try to diminish the risk of re-neovascularisation during re-do operation. Effectively, another re-do in the groin could be quite impossible. Consequently we must keep in mind a re-do must be the last one.
In a recent study De Maeseneer [41] found that patch saphenoplasty significantly improved the clinical and duplex ultrasound results five years after repeat surgery. Recurrent thigh varicosities were observed in 58% of limbs without a patch and in 26% with a patch. After 5 years Duplex scans revealed important neovascular vessels in 45% in the group without a patch versus 9% in those with a patch (P=.0007). The use of a silicone patch at repeat operation to treat recurrent varicose veins is associated with a lower incidence of neovascularisation. In re-do procedures, it is of particular importance to fix the patch well to the CFV, as there is usually no cribriform fascia left to cover the patch and keep it where it should stay (covering the saphenous stump).
- In another randomised trial [42] of PTFE patch insertion for re-do 32 legs attended for assessment at 2 years. Four of 16 legs without a patch and 5 of 16 legs with a patch developed neovascularisation. The author concludes that PTFE patch did not appear to contain neovascularisation.
- Between 1991 and 1999 we were used to carrying out a patch saphenoplasty for surgery of recurrent sapheno femoral incompetence. This study reported 137 (100 patients) [43]. Five-year follow-up was obtained for 119 legs.
The re-do surgical procedures were done with the patient under locoregional anesthesia, with a femoral nerve block, injection of 50% lidocaine solution (1%) to extend the area of anesthesia to residual varicose veins. Eighty-two percent of patients chose to be treated as outpatients; the others were discharged the day after surgery. The operation was done by using a method similar to that employed in endoscopic procedures, with a lateral approach involving an incision placed at a distance remote from the femoral vein and minimal tunnel dissection to expose the stump of the tied vein. A 4-cm oblique lateral skin incision was made, with the medial end above the residual stump that had been marked preoperatively with use of Doppler scanning. Dissection directly to the femoral vein was accomplished by using binocular loupes without an electric scalpel. The vascular sheath was opened, and the residual stump was dissected by raising the prevascular flap forward. After initial suture ligation of the stump flush with the femoral vein, dissection was continued distally to the division branches of the residual stump. Collaterals were divided after placement of clips. If present, the isolated trunk or residual great saphenous trunk was pre-tied for stripping. Obliteration of the stump was achieved by burying it under its lumen with a back-and-forth suture, laying the stump over the adventitia of the femoral vein. A partition was made between the stump suture and the tied collaterals in the prevascular subcutaneous tissues by interposing an ePTFE patch (0.1 mm thick, 1 cm long and 1.5 cm wide; Preclude Peritoneal Membrane, W.L. Gore & Associates, Flagstaff, Arizona, USA). The patch was attached by applying biological glue under it and in the dissection space. To facilitate placement and avoid displacement of the patch, the dissection space was made as obliquely and as small as possible (Photo 3). No suction drains were inserted. The incision was closed with intradermal resorbable sutures.
Only 5 extremities (4.2%) had poor results, with varices and a new connection in the same site as previously and theoretically requiring another re-do procedure in the groin. Two of the 5 failures occurred in obese patients, including the only patient who had an abscess. Another failure was due to insufficient resection.
It is surprising to compare my personal results using a very small patch (1/1.5 cm) (4% of re-neovascularisation at 5 year follow-up) [43] with those of other good surgical teams [41, 42]. It is known of their comprehensive large experience in re-do surgery, (31% at 2 year follow-up for Winterborn, 9% and 22.3% for De Maesseneer). The differences are also in the rate of complications (0.7% in my personal study with no lymphatic and hematoma complication and only one postoperative abscess, 32.5 and 19% for Winterborn and De Maesseneer respectively). In fact, in these studies many complications are reported, 13%, 6% infections, 6.5%, 3% hematomas, 13%, 10% lymphatic complications and 0.9% stenosis of the common femoral vein [44]. Regarding these studies and Earnshaw’s [35] as well and specially the pictures and photographs of the operations we can see that the dissection was always large with a wide exposure of the femoral vein. This can explain exceptional late postoperative stenosis of the common femoral vein reported in recent series [44].
I am convinced that neovascularisation are produced by the complications which are induced by a large dissection. It is likely in my study [43] the low rate of neovascularisation (4%) was not due to the patch but to the low rate of complications, itself due to the small dissection.
Consequently since 2000 we have still reduced the aggressiveness of re-do operations by doing only a ligature of the stump with non absorbable suture through the same small lateral approach (Photo 4). Usually the ligature of the stump is easy because there is always continuity between the adventitia of the femoral vein and the residual stump. Finally, intra-operatively, I securely inject foam in the distal part of the ligated stump from top to bottom (Photo 5) or by the way of a thigh vein from bottom to top (Photo 6). From top to bottom the puncture of the vein must be performed carefully with a 25 gauge needle and a prolongator in order not to move. The reflux is verified. During the injection we can see the whitening of the injected vein. The foam is prepared preoperatively according to Tessari method [45] with 2 sterile syringes without silicon and a 3-way stopcock. The aim of the surgical ligature of the stump is to secure and make the foam sclerotherapy effective avoiding the passage of the foam in the common femoral vein.
CONCLUSION
Regarding the neovascularisation the surgeon is not an innocent bystander. There are some gestures, habits which can be unfortunately responsible for the appearance of neovascularisation. Do not touch the SFJ if we want to avoid any risk of neovascularisation! Firstly, after a comprehensive ultrasound scanning of the SFJ doing nothing when there are no incompetence of terminal and pre-terminal valves. When there is only an incompetent preterminal valve it is better to choose endovascular technique like EVL or RFO. When a flush ligation is decided it is mandatory to avoid useless dissection, wrenching, bruising, retractor gapping, bleeding, electrocoagulation, postoperative haematomas and other complications. The larger the incision the worse the neovascularisation. On the contrary, extensive resection of all incompetent residual trunks and varices on the thigh are mandatory. Concerning re-do surgery it is mandatory to avoid useless re-do, the best choice is foam sclerotherapy. The barrier technique are, probably, not so useful as we was told 10 years ago. The lack of aggressiveness during the operation is certainly far more important: “Doing less in the groin to do better for the recurrence and re-recurrence”
REFERENCES
- De Maesseneer MG. Neovascularisation: an adverse response to proper groin dissection. in : Bergan JJ (ed), The Vein Book, San Diego CA USA, 2007, 239-246
- De Maesseneer MG. Strategies to minimize the effect of neovascularization at the saphenofemoral junction after great saphenous vein surgery: an overview. Phlebolymphology. 2006 ;13 : 205-20
- Von Langenbeck B. Beitrage zur chirurgischen Pathologie der Venen Arch Klin Chir 1861; 1: 47
- Glass GM. Neovascularization in recurrence of the varicose great saphenous vein following Transection. Phlebology. 1987;2: 81-91.
- Schmid-Schönbein GW. Molecular basis of venous insufficiency. In : Bergan JJ (ed), The Vein Book, San Diego CA USA, 2007, 67-78
- Shoab SS, Scuit JH, Coleridge-Smith PD. Increased plasma vascular endothelial growth factor among patients with chronic venous disease.J Vasc Surg. 1998;28: 535-540
- Hollingsworth SJ, PowelI GL, Barker SGE, Cooper DG. Primary varicose veins: Altered transcription of VGFE and its receptors (KDR, flt-1, soluble flt-1) with sapheno-femoral junction Incompetence. Eur J Vasc Endovasc Surg. 2004; 27: 259-268
- Rewerk S, Labretsas K, Winkler M, Nüllen H, Duczek C, Meyer AJ, Grobholtz R, Thomas N. Vascular endothelian grouth factor (VEGF) and VEGF-receptor (VEGF-R) in the pathogenesis of primary and recurrent varicose. Phlebolymphology 2007; 14:154 (abstract)
- Takase S, Schmid-Schonbein G, Bergan. Leukocyte activation in patients with venous insufficiency J Vasc Surg 1999;30: 148156
- Raffetto JD. Chronic venous insufficiency: molecular abnormalities and ulcer formation. in : Bergan JJ (ed), The Vein Book, San Diego CA USA, 2007, 79-87
- El Wajeh Y, Giannoukas AD, Gulliford CJ, Suvarna SK, Chan P. Saphenofemoral venous channels associated with recurrent varicose veins are not neovascular. Eur J Vasc Endovasc Surg. 2004 ;28:590-4.
- Takase S, Lerond L, Bergan JJ. Schmid-Schonbein GW. The inftammatory reaction during venous hypertension in the rat. Microcirculation. 2000;7: 41-52
- Owatverot TB, Oswald SJ, Chen Y, Wille IJ, Yin Fe. Effect of combined cyclic stretch and fluid shear stress on endothelial cellmorphological responses, J Biomech Eng. 2005;127: 374-382.
- Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, Wofovitz E. Fluid shear stress and the vascular endothelium:For better and for worse, Prog Biophys Mol Biol. 2003; 81: 177199.
- Abumiya T, Sasaguri T, Taba Y, Miwa y, Miyagi M. Shear stress induces expression of vascular endothelial growth factor receptor (Flk-1/K.DR) through the CT-rich Sp 1 binding site. Arterioscler Thromb Vasc Biol. 2002; 22: 907-913.
- Coleridge Smith PD. Leg Ulcers: Biochemical Factors. Phlebology. 2000;15: 156-161.
- Lefebvre-Vilardebo M. Vous avez dit « Néovascularisation inguinale post-chirurgicale »? Editorial. Phlébologie 2001; 54: 253-254
- Munasinghe A, Smith C, Kianifard B, Price BA, Holdstock JM, Whiteley MS. Strip-track revascularization after stripping of the great saphenous vein. Br J Surg. 2007;94:840-3
- Mitchel G, Rosser S, Edwards PR, Dimitri S, de Cossart L. Vascularisation of the haematoma tract following long saphenous vein stripping: a new cause of recurrent varicose veins. Phlebology 2003;18:48 (abstract)
- Earnshaw JJ, Davies B, Harradine K, Heather BP. Preliminary results of PTFE patch saphenoplasty to prevent neovascularisation leading to recurrent varicose veins Phlebology 1998;13:10-3
- Glass GM. Neovascularization in recurrence of varices of the great saphenous vein in the groin: Surgical anatomy and morphology. Vascular Surgery. 1989;23: 435-442
- Glass GM. Neovascularization in recurrent sapheno-femoral incompetence of varicose veins: Surgical anatomy and morphology.Phlebology. 1995; 10: 136-142
- van Rij AM, Jones GT, Hill GB, Jiang P. Neovascularization and recurrent varicose veins: More histologic and ultrasound evidence. J Vasc Surg 2004;40: 296-302
- Nyamekye I, Shephard NA, Davies B, Heather BP, Earnshaw JJ. Clinicopathological evidence that neovascularisation is a cause of recurrent varicose veins. Eur J Vasc Endovasc Surg; 1998. 15: 412415
- Stücker M, Netz K, Breuckmann F, Altmeyer P, Mumme A. Histomorphologic classification of recurrent saphenofemoral reflux. J Vasc Surg 2004; 39: 816-821
- Frings N, Nelle A, Tran P, Fischer R, Krug W. Reduction of neoreflux after correctly performed ligation of the saphenofemoral junction. A randomized trial. Eur J Vasc Endovasc Surg. 2004;28:246-252.
- Haas E, Burkhardt T, Maile N. Rezidivhäufigkeit durch Neoangiogenese nach modifizierter Krossektomie. Phlebologie. 2005;34:101-104.
- Jones L, Braithwaite BD, Harradine K, Earnshaw JJ. Neovascularization is the principal cause of varicose vein recurrence: results of a randomized trial of stripping the long saphenous vein. Eur J Vasc Endovasc Surg 1996;12:442-445
- Egan B, Donnelly M, Bresnihan M, Tierney S, Feeley M. Neovascularization: an « innocent bystander » in recurrent varicose veins. J Vasc Surg. 2006;44:1279-84
- Glass GM. Prevention of recurrent saphenofemoral incompetence after surgery for varicose veins Br J Surg. 1989;76:1210.
- Glass GM. Prevention of the sapheno-femoral and sapheno-popliteal recurrence of varicose veins by forming a partition to contain neovascularization. Phlebology 1998;13:3-9
- Sheppard M. A procedure for the prevention of recurrent saphenofemoral incompetence. Aust N Z J Surg. 1978;48: 322-326.
- Gibbs PJ, Foy DM, Darke SG. Reoperation for recurrent saphenofemoral incompetence: a prospective randomized trial using a reflected flap of pectineus fascia. Eur J Vasc Endovasc Surg. 1999;18:494-498.
- De Maeseneer MG, Giuliani DR, Van Schil PE, De Hert SG. Can interposition of a silicone implant after sapheno-femoral ligation prevent recurrent varicose veins? Eur J Vasc Endovasc Surg. 2002;24:445-449.
- Earnshaw JJ, Davies B, Harradine K, Heather BP. Preliminary results of PTFE patch saphenoplasty to prevent neovascularization leading to recurrent varicose veins. Phlebology. 1998;13:10-13.
- De Maeseneer MG, Philipsen TE, Vandenbroeck CP, Lauwers PR, Hendriks JM, De Hert SG, Van Schil PE. Closure of the cribriform fascia: an efficient anatomical barrier against postoperative neovascularisation at the saphenofemoral junction? A prospective study. Eur J Vasc Endovasc Surg. 2007;34:361-6
- Pichot O, Sessa C, Bosson JL.Duplex imaging analysis of the long saphenous vein reflux: basis for strategy of endovenous obliteration treatment. Int Angiol. 2002 ;21:333-6.
- Cappelli M, Molino Lova R, Ermini S, Zamboni P.Hemodynamics of the sapheno-femoral junction. Patterns of reflux and their clinical implications. Int Angiol. 2004;23:25-8.
- Kianifard B, Holdstock JM, Whiteley MS. Radiofrequency ablation (VNUS closure) does not cause neo-vascularisation at the groin at one year: results of a case controlled study. Surgeon 2006;4:71-4
- Lurie F, Creton D, Eklof B, Kabnick Ls, Kistner Rl, Pichot O, Sessa C, Schuller-petrovic S. Prospective Randomized Study of EndoVenous radiofrequency Obliteration (Closure) versus Ligation and Vein Stripping (EVOLVeS): two-year follow-up. Eur J Vasc Endovasc Surg 2005;29:67-73
- De Maeseneer MG, Vandenbroeck CP, Van Schil PE. Silicone patch saphenoplasty to prevent repeat recurrence after surgery to treat recurrent saphenofemoral incompetence: long-term follow-up study. J Vasc Surg. 2004;40:98-105.
- Winterborn RJ, Earnshaw JJ. Randomised trial of PTFE patch insertion for recurrent great saphenous varicose veins. Eur J Vasc Endovasc Surg. 2007 ;34:367-73
- Creton D. Surgery for recurrent saphenofemoral incompetence using expanded polytetrafluoroethylene patch interposition in front of the femoral vein: long-term outcome in 119 extremities. Phlebology. 2002;16:93-97.
- De Maesseneer MG, Vandenbroeck CP, Lauwers PR, Hendricks JM, De Hert SG, Van Schil PE. Early and late complications of silicone patch saphenoplasty at the saphenofemoral junction. J Vasc Surg. 2006 ;44:1285-90
- Tessari L, Cavezzi A, Frullini A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg. 2001;27:58-60
Table I
The role of the surgeon. Among the different causes of neovascularisation there are 3 which depend directly on the surgeon (2, 3, and 6).
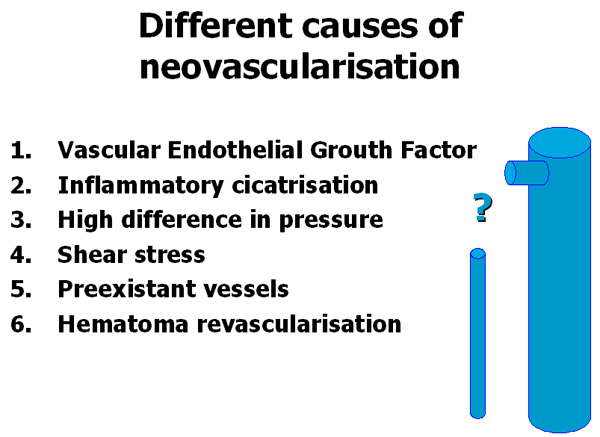
Photo 1
When the terminal valve and the tributaries must be protected a section below the triburaries is performed.
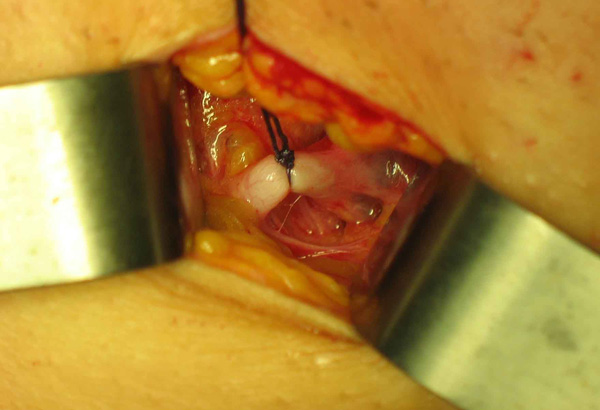
Photo 2 & 3
In the case of incompetent anterior accessory saphenous vein with competent terminal and preterminal valves only a section of the anterior accessory saphenous vein is necessary.
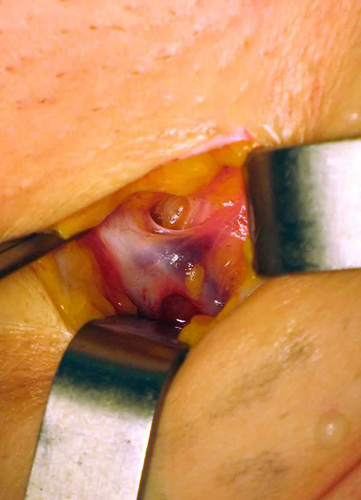
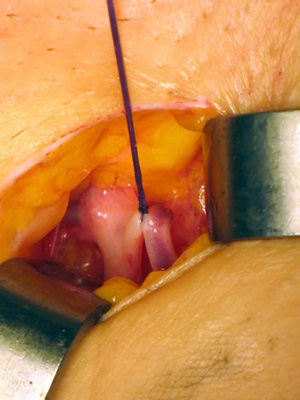
Photo 4
the dissection space was made as obliquely and as small as possible.
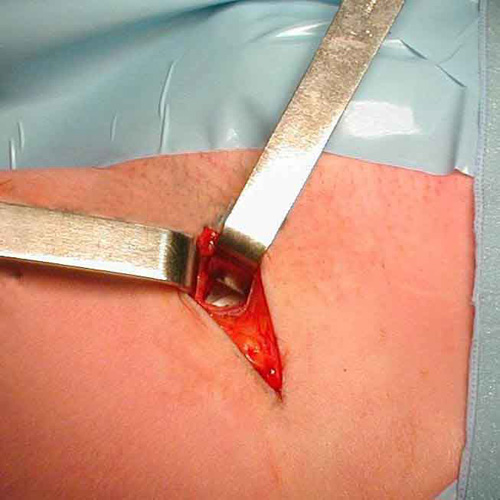
Photo 5
A ligature of the stump with non absorbable suture is carried out through the same small lateral approach.
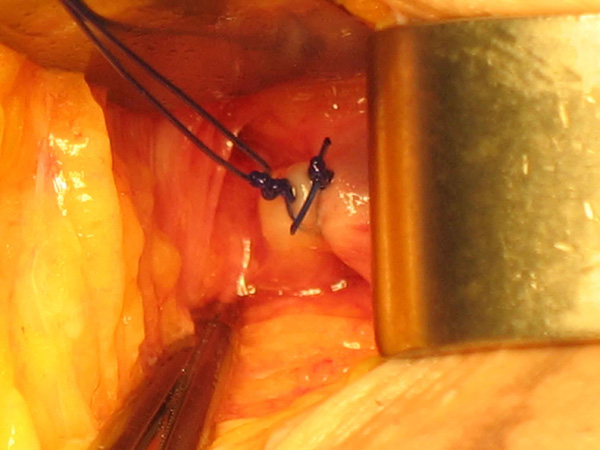
Photo 6
The injection of foam in the distal part of the ligated stump from top to bottom is carried out carefully with a 25 gauge needle and a prolongator in order not to move.
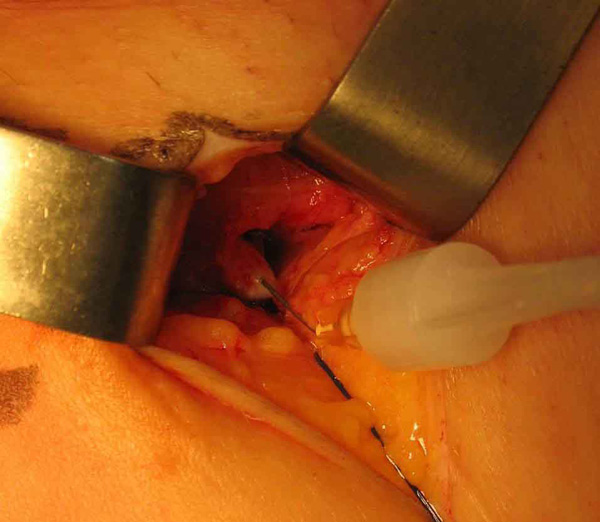
Photo 7
The injection of foam is carried out by the way of a thigh vein from bottom to top.
| Date | 2008 |
| Auteurs | Phlebologie 2008;37:134-141 D. CRETON Denis Creton, MD, EC A Paré, rue A Paré, 54100 F, Nancy, France - Telephone: 33 3 83 95 54 00; fax: 33 3 83 95 54 23; e-mail: [email protected]. |






