Foam Sclerotherapy Combined with Surgical Treatment for Recurrent Varicose Veins: Short Term Results

Foam Sclerotherapy Combined with Surgical Treatment for Recurrent Varicose Veins: Short Term Results
Objective. To study the short term results of combined peroperative foam sclerotherapy (PFS) and surgical treatment for recurrent varicose veins.
Methods. PFS was used to treat 129 limbs with recurrent varices: 100 great saphenous (GSV), 29 small saphenous veins (SSV). Foam was prepared with 1% polidocanol mixed with 4 times its volume of air. The 100 GSVs comprised 28 trunks directly connected with the femoral vein, 28 connected to a lymph node venous network, 11 associated with perforators and 33 isolated trunks. The 29 SSVs comprised 4 trunks directly connected to the popliteal vein, 7 isolated trunks, 15 popliteal perforators and 3 recanalisations after SSV stripping. All operations included phlebectomies. In twenty limbs re-ligation of the SFJ and 4 SPJs was carried out. All were performed under local anaesthesia in an ambulatory setting. Patients were assessed clinically and by colour duplex ultrasound after 3 and 40 days follow-up.
Results. 120 patients (93%) showed complete obliteration of saphenous trunks, junctions and varices. The 9 incomplete obliterations were 3 venous recanalisations in the SSV compartment and 6 perforators (4 popliteal and 2 femoral). Two asymptomatic deep venous thromboses were detected by colour duplex 3 days after operation.
Conclusion. PFS facilitates surgical treatment of recurrent varicose veins. There is a small risk of post-operative deep vein thrombosis.
Introduction
Foam sclerotherapy has become widely used in recent years and one randomised study has found that the medium term outcome is similar to that of surgery.1 It has been shown that foam sclerosants are more effective than the same volume of liquid sclerosant of similar strength.2 There is a European consensus that foam sclerotherapy may be carried out safely and effectively for the management of varicose veins, although a wide range of strategies is currently employed for the use of foam.3,4
The modern evolution of varicose vein surgery has been towards less traumatic techniques. However, surgery for recurrence unfortunately often necessitates aggressive dissection or inadequate treatment may result.5 Foam sclerotherapy may play a role in the management of recurrent varicose veins by replacing the more traumatic parts of the operation.
Since 2001 we have used peroperative foam sclerotherapy (PFS) to complete the more invasive parts of the surgical procedures. In particular we have used PFS instead of stripping or re-ligation of recurrent junctions with the aim of minimising the extent of dissection. We consider that it is undesirable to extend the duration of treatment by using postoperative sclerotherapy and have aimed to treat all aspects of recurrent varices in a single treatment. A previous study6 demonstrated that foam sclerotherapy could be used for about 30% of the patients undergoing redo surgery.
The main advantage of using foam sclerotherapy during surgery is the opportunity to introduce the sclerosant directly into the recurrent varices, trunk or junction under direct vision. If no vein is readily exposed surgically, an injection under ultrasound guidance may be used instead. This improves on what may be achieved by surgery in two ways. Firstly, veins which are difficult or impossible to manage surgically can be treated, including deeply located venous networks or recurrence within the saphenous compartment as well as residual saphenous trunks. Secondly complex networks of veins in the pudendal region or femoral lymph node are readily managed without the risks normally associated with surgery. The risk of damage to nearby nerves and lymphatics is reduced by this strategy.
Material and Methods
Patients
Between June 2003 and April 2005, 129 unselected patients underwent foam sclerotherapy during surgery for varicose vein recurrence. Of these, only 19 had been previously operated on by the same surgeon (DC), 91 had been operated on by other surgeons and no data was recorded for the remainder. There were 105 women (81%) and 24 men (19%); only one leg was treated in each patient (81 left sides and 48 right sides). The mean age was 55 years (range 29e76). Limbs were assigned to the appropriate CEAP classification as well as the REVAS5 additions to CEAP. These data are shown in Tables 3.
Venous duplex ultrasound examination was carried out with the patients in a standing position (General Electric Kretz Expert 730, 7/13 MHz transducer, 78457 Velizy, France). Reflux was elicited by a manual calf compression-release manoeuvre. Reflux exceeding 0.5 seconds was considered significant. The diameter and the length of the incompetent communication between the deep and the superficial system was recorded along with the location, length and diameter of the incompetent residual saphenous trunk. The aim of ultrasound investigation was to select the venous segments where foam sclerotherapy was preferred since surgical treatment may have resulted in risks to adjacent structures or been incomplete.
Prior to treatment all patients gave their informed consent to the proposed treatment and follow-up. Immediately before surgery a further ultrasound examination was performed (Esaote AU4 Idea, Le Perreux, France, 10 MHz transducer) to identify the best points for the injection of foam.
Surgery was performed under tumescent local anaesthesia or femoral block in an ambulatory setting. The aim of the operation was the closure of incompetent communications between the deep and superficial venous system combined with the removal (or closure) of incompetent residual trunks and the ablation of varices. Varices were generally considered best managed by phlebectomy, whereas residual incompetent trunks, perforators and inguinal or popliteal recurrences were considered for either surgical treatment or foam sclerotherapy according to the expected technical difficulty of surgery. Any part of the treatment which was attempted surgically but required excessively aggressive dissection was managed by foam sclerotherapy. The number of phlebectomy incisions, corresponding to the extent of varices treated, was recorded.
Sclerosant foam was made with 1% polidocanol (AetoxisclerolÒ, Kreussler, Germany) using the Tessari method.7 Two ml of Polidocanol were mixed with four times the volume of air (8 ml) to produce 10 ml foam. Foam was made during the operation immediately before injection.
After foam injection local compression was applied over treated veins for one minute, as long as this did not hinder ultrasound imaging of the limb. The foam volume injected was 2e4 ml for the SSV and 4e6 ml for the GSV. Although the risk of skin necrosis after foam is small, the vein or trunk in which foam had been injected was always ligated to minimise extravasation. Inguinal compression was performed to prevent the foam from entering the femoral vein (compression of the saphenous femoral junction during foam injection into the saphenous trunk: Cloutier’s Manoeuvre). The limb was raised in order to facilitate distal progression of foam and to minimise passage of foam into the deep venous system.
The technique of foam introduction was dependant on the location and topography of the network to be treated :
The injection of any « lymph node venous network », also known as the « dystrophic venous network of the groin »,8 was performed in a downward direction under visual control by direct needle puncture through the redo incision in the groin (Fig. 1). Deep injections at the bottom of the incision were facilitated by using a long 22 g needle and a 25 cm extension tube. Distal to the groin incision ultrasound-guided puncture (needle or Butterfly) or injection through a long catheter with its tip located at the proximal end of the residual trunk were used. Surgical intervention was used for recurrence at the SFJ where the diameter was greater than 3 mm. Two types of disconnection of the residual SFJ were carried out: ligation-section with patch interposition or ligation only using a nonabsorbable suture material. In order to avoid the risk of common complications of re-do surgery in the groin (neovascularization, neurological and lymphatic lesions) we chose to carry out a lateral approach in the groin to avoid areas of previous dissection and used binocular loupes.9 The aim of this ligation was to avoid passage of foam into the femoral vein during injections near the groin. In cases where there was a long residual stump at the SPJ, flush ligation with the popliteal vein was carried out.
Recanalisation of venous pathways such as previously stripped great and small saphenous veins were treated by ultrasound guided injection with foam. These were invariably complex tangled networks of veins lying in the saphenous compartment of the previously stripped vein. Effective surgical treatment of these veins is impossible (Fig. 2).
Sclerosis of a residual saphenous trunk was easily carried out by inserting a long catheter, (angiocath 4F, Super Torque, Johnson&Johnson Company, Cordis Europa NV, Oosteinde 8, NL-9301 L J Roden) through the part of the trunk that had been exposed during phlebectomy. When the residual trunk could not be reached by phlebectomy, sclerosis was performed under ultrasound guidance with a 22 G needle or a Butterfly (Microflex, Vygon BP7, 95440 F-Ecquen) equipped with an extension tube. This allowed aspiration and saline rinsing manoeuvres without moving the needle. During the foam injection by means of a catheter, the correct positioning of the tip of the catheter was achieved by ultrasound imaging. Usually the catheter was visible with a 10 or 13 MHz probe. Saline injection was use to facilitate visualisation of the vein around the catheter The catheter was filled with saline before insertion into the vein to facilitate blood flow into the catheter and to minimise injection of air bubbles from the catheter. The foam injection was usually performed as the catheter was pulled out.
Perforator injection was carried out either by direct puncture or indirectly through a long catheter where the perforator was connected to a residual saphenous trunk. When the perforator was connected with varicose veins that could be accessed by a phlebectomy incision, foam injection was easily performed through a small 20 G catheter (OptivaÒ 2 Medex Medical Ltd, Rossendale, BB4 4PW, UK).
Post operative compression was achieved by application of two class II French stockings (2 Â 20 1⁄4 40 mm Hg) for two days and then by one stocking for 2 weeks. Bandages were not used. Patients could choose either ambulatory surgery with return home on the same day as treatment or hospitalization which was more appropriate for patients living alone or where no one was available to escort them home. No restriction of daily activities was advised. No analgesic drugs were prescribed postoperatively although patients were allowed to take proprietary drugs obtained without prescription from a pharmacy. Thrombopropylactic injections of enoxaparine 20 mg (LovenoxÒ, Sanofi Aventis, France) were given to all patients by a nurse at home for 8 days.
The patients were examined 3 days and 40 days following PFS. Clinical and duplex ultrasound investigations were performed on each occasion. The aim these examinations was to assess the deep veins for the presence of deep venous thrombosis (DVT) and to evaluate efficacy of sclerosis of veins. Well established efficiency criteria were used3 and included the presence of an incompressible vein, absence of reflux on colour Doppler when performing a Valsalva manoeuvre and calf compression/release manoeuvre. Extension of thrombophlebitis into deep veins, possible residual reflux, haematomas and residual varices were also sought.
As the follow-up period of 40 days was very short the patients were asked to answer only one question: « Are you feeling better, the same or worse than before ». Clinical evaluation for the development of new varices was not done since the very early stage of follow-up was too early for any further varices to be clinically apparent.
Results
One hundred recurrences in the GSV territory and 29 in the SSV territory were treated. All operations consisted of phlebectomies with or without inguinal or popliteal fossa redo operations. The mean number of phlebectomy incisions was 24.3 (range 8e82).
Distribution of the type of PFS
Recurrence after previous GSV surgery represented most of the indications. The type of sclerotherapy depended on the anatomical type of these 100 cases (Fig. 3).
There were 28 residual trunks directly connected with the femoral vein via a residual saphenofemoral junction. Among these, only 16 inguinal redo operations were performed before the injection, in order to divide (n 1⁄4 8) or ligate with a non absorbable suture (n 1⁄4 8) the new communication between the femoral vein and the varices. The 12 remaining trunks underwent foam injection only without surgical disconnection.
Twenty-eight residual trunks connected with the femoral vein through new vessels, « lymph node venous network » or sometimes without any clear connection with the deep system, were sclerosed with a catheter introduced from below.
Thirty-three isolated residual trunks without any connection with the deep venous system were treated by foam injection carried out through a catheter or by direct needle puncture.
Eleven long thigh perforators with small diameters connected to a residual trunk were injected through a catheter or by direct puncture.
The 29 recurrences after SSV surgery were treated according to the type of recurrence (Fig. 4) :
Fifteen popliteal fossa perforators were treated with foam injection by direct puncture without redo operation in the popliteal fossa.
Four residual trunks directly connected with the popliteal vein via a long residual stump were sclerosed during operation just after flush ligation of the long residual stump.
Three cases of SSV recanalisation were sclerosed by direct puncture.
In 7 cases, a residual SSV without any connection with the popliteal vein was injected via a long catheter.
Results at follow-up
No patient presented with either any visual disturbance, migraine, neurological complications or local complications such as necrosis or cutaneous matting. No significant post-operative complication occurred following surgical re-ligation of the SFJ or SPJ. There was no case of lymphatic leak and no patient reported symptoms suggestive of nerve injury. No case of wound infection was encountered.
Fourteen patients failed to attend for the 3 day follow-up but all attended at 40 days. Clinical results at 40-days were excellent. Varicose veins were completely eliminated improved quality of life; 94 patients felt better, 25 felt similar and 6 felt worse as a consequence of subcutaneous postoperative haematomas, 4 no data.
Two thigh perforators (out of 11) and four perforators in the popliteal fossa (out of 15) were not completely closed. Three venous recanalisations in the saphenous compartment after stripping of the SSV were not completely closed. Obliteration of treated veins was complete in 92% of patients at the 40-day follow-up.
Two clinically asymptomatic deep vein thromboses were discovered at the first ultrasound examination on day 3. Partial thrombosis of the femoral vein arising from the inguinal connection of a sclerosed saphenous trunk occurred in one case. This residual trunk was one of 12 directly connected with the femoral vein by a residual stump. This trunk was injected without prior ligation or disconnection. The second was a gastrocnemius vein thrombosis after the sclerosis of a SSV residual trunk (one of 7). These 2 postoperative thromboses were successfully treated by a course of low molecular weight heparin.
Discussion
At the 40 day follow-up a small number of veins were found to have recanalised despite treatment by foam sclerotherapy. Our aim of avoiding the need for post-operative treatment for residual veins was therefore not completely achieved. The percentage of complete obliterations after injection (92%) corresponds to the percentage mentioned in the literature regarding sclerotherapy for primary varices10,11,12 and for recurrence.13 The 9 cases of incomplete obliteration probably result from inadequate filling of veins with foam, perhaps due to our limited experience of this technique and our fear of causing damage to the deep veins by injecting an excessive volume.
The three recanalisations that occurred following treatment of recurrences in the track of the stripped SSV involved large veins 6 or 7 mm in diameter. These probably required much more foam than we injected. Similarly, large popliteal perforators or thigh perforators connected directly to the deep veins (femoral or popliteal), were not completely filled to avoid excess foam reaching the deep veins. These types of perforators are often subjected to high venous pressures increasing the tendency to reopen. Successful treatment of these vein types may require better emptying of the veins by limb elevation and injection of larger volumes of sclerosant foam.
The peroperative use of foam makes it more efficient, because the trunks are first surgically disconnected. Since there is no blood flow following ligation the foam is neither displaced nor diluted, enhancing the efficacy of treatment. Combined phlebectomies considerably reduce the risk of superficial venous thrombosis in areas treated by sclerotherapy. The efficiency of foam sclerotherapy is also strongly influenced by phlebectomies which leads to abolition of reflux in the residual saphenous trunks.
In this study, we observed two cases of DVT which where probably due to the fact that most patients did not get up and walk immediately after sclerotherapy or got up only after a thirty-minute rest period. No « venous flush exercises » were performed.13 At the GSV level, a single thrombosis occurred for a trunk directly connected to the femoral vein. This appeared to arise by extension of thrombus from the saphenous trunk. Surgical exclusion should have been performed first.
Thromboembolic risk seems to increase when sclerosis is performed per-operatively probably due to an activation of coagulation factors during surgery14,15,16 No data is available concerning the DVT risks after varicose vein surgery carried out under local anaesthesia in ambulatory setting. Clinically apparent thromboembolic complications occurred in 0.43% of 4206 patients treated surgically for varicose veins under loco-regional anaesthesia without LWMH prophylaxis.17 In this series we used post-operative LMWH prophylaxis in the hope of minimising the risk of DVT. This strategy is not unanimously accepted4 since it may impair the efficacy of sclerotherapy.18
This clinical series shows the feasibility of using foam sclerotherapy during surgical treatment for recurrent varices. This led to a high rate of obliteration of residual saphenous trunks, recurrent varices and recanalisations of previously stripped veins. We consider that it is less hazardous than aggressively pursuing technically difficult surgical approaches which may result in lymphatic leaks or nerve injury. Two cases of DVT in our series show that even though foam sclerotherapy minimises the extent of surgery for recurrent varicose veins, thrombotic complications may arise despite prophylactic heparin treatment.
Acknowledgements
We thank Ms Marie Jose Schantz Fleurent and Dr Dominique Vala for their technical help in editing the text for the English language.
REFERENCES
- WRIGHT D, GOBIN JP, BRADBURY AW, COLERIDGE-SMITH P, SPOELSTRA H, BERRIDGE D et al. The VarisolveÒ European Phase III Investigators Group. VarisolveÒ polidocanol microfoam compared with surgery or sclerotherapy in the management of varicose veins in the presence of trunk vein incompetence: European randomized controlled trial. Phlebology 2006;21:180e190.
- HAMEL-DESNOS C, DESNOS P, WOLLMANN JC, OUVRY P, MAKO S, ALLAERT FA. Evaluation of the efficacity of polidocanol in the form of foam compared with liquid foam in sclerotherapy of the great saphenous vein: initial results. Dermatol Surg 2003;29:1170e1175.
- BREU FX, GUGGENBICHLER S. European consensus meeting of foam sclerotherapy. Dermatol Surg 2004;30:709e717.
- RABE E, PANNIER-FISCHER F, GERLACH H, BREU FX, GUGGENBICHLE S, ZABEL M. German Society of Phlebology. Guidelines for sclerotherapy of varicose veins. Dermatol Surg 2004;30:687e693.
- PERRIN MR, GUEX JJ, RUCKLEY CW, DE PALMA RG, ROYLE JP, EKLOF BO et al. and the REVAS group. Recurrent varices after surgery (REVAS), a consensus document. Cardiovasc Surg 2000;8:233e245.
- CRETON D. La gestion de la recidive apres chirurgie des varices des membres inferieurs: chirurgie ou sclerotherapie? Phlebologie 2004;57:317e326.
- TESSARI L, CAVEZZI A, FRULLINI A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg 2001;27:58e60.
- LEMASLE P, UHL JF, LEFEBVRE-VILARDEBO M, GILLOT C. Les reseaux veineux dystrophiques de la lame ganglionnaire du scarpa. Phlebologie 1999;52:263e270.
- CRETON D. Surgery for recurrent saphenofemoral incompetence using expanded polytetrafluoroethylene patch interposition in front of the femoral vein: long-term outcome in 119 extremities. Phlebology 2002;16:93e97.
- BARRETT JM, ALLEN B, OCKELFORD A, GOLDMANN MP. Microfoam ultrasound-guided sclerotherapy of varicose veins in 100 legs. Dermatol Surg 2004;30:6e12.
- FRULLINI A, CAVEZZI A. Sclerosing foam in the treatment of varicose veins and telangiectases:history and analysis of safety and complications. Dermatol Surg 2002;28:11e15.
- SADOUN S, BENIGNI JP, SICA M. Etude prospective de l’efficacite de la mousse de sclerosant dans le traitement des varices tronculaires des membres inferieurs. Phlebologie 2002;55:259e262.
- FRULLINI A. Sclerosing foam in the treatment of recurrent varicose veins. In: HENRIET JP, ed. Foam sclerotherapie State of art. Paris: Editions Phlebologiques Francaises; 2002:73e78.
- IWAMOTO S, IKEDA M, KAWASAKI T, MONDEN M. Treatment of varicose veins: an assessment of intraoperative and post-operative compression sclerotherapy. Ann Chir Vasc 2003;17:290e295.
- IKEDA M, KAMBAYASHI J, IWAMOTO S, SHINOKI N, NAKAMURA T, OKAHARA K et al. Hemostasis activation during sclerotherapy of lower extremity varices. Thromb Res 1996;82:87e95.
- YAMAKI T, NOZAKI M, SASAKI K. Acute massive pulmonary embolism following high ligation combined with compression sclerotherapy for varicose veins. Dermatol Surg 1999;25:321e325.
- LEMASLE P, LEFEBVRE VILARDEBO M, UHL JF. Faut-il vraiment prescrire des anticoagulants apres chirurgie d’exerese des varices? Resultats d’une etude prospective portant sur 4 206 interventions. Phlebologie 2004;57:187e194.
- CAVEZZI A, FRULINI A, RICCI S, TESSARI L. Treatment of varicose veins by foam sclerotherapy: two Clinical Series. Phlebology 2002;17:13e18.
Fig. 1
GSV injection can be performed downwards under visual control by direct puncture through a surgical incision or upwardly by means of a catheter or by an ultrasound-guided puncture.
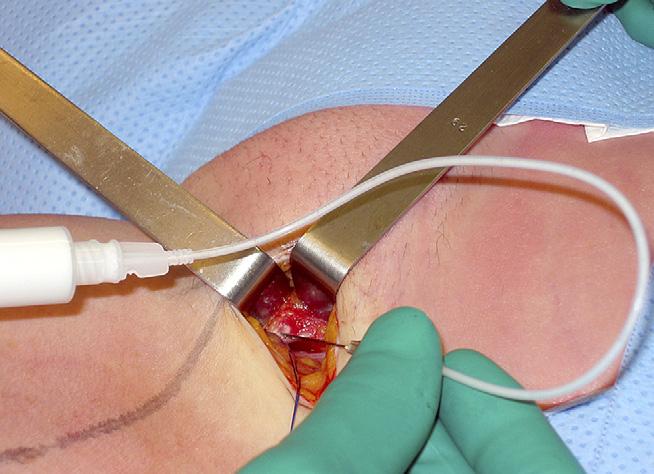
Fig. 2
Recanalisation after stripping between two portions of a residual trunk on the small saphenous vein.
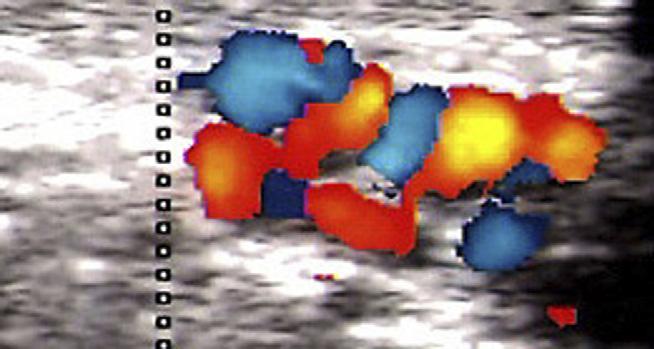
Fig. 3
Distribution of the 100 operation for GSV recurrence : 28 residual trunks connected to the femoral vein through a residual sapheno-femoral junction (SFJ) (16 underwent redo surgery in the groin. 8 ligation-section and 8 ligation only). 61 residual trunks (33 isolated and 28 connected to the GSV) and 11 perforators. Arrows indicate the location of foam injection.
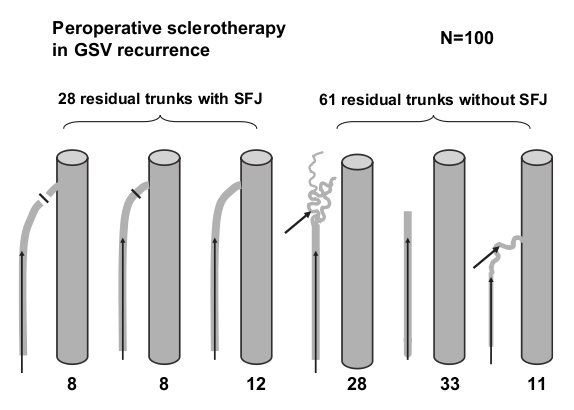
Fig. 4
Distribution of the 29 PFS in SSV recurrence: 4 residual trunks connected to a long residual stump (redo surgery in popliteal fossa), 15 perforators in the popliteal fossa, 7 isolated residual trunks and 3 recanalisations in the saphenous compartment. Arrows indicate the location of foam injection.
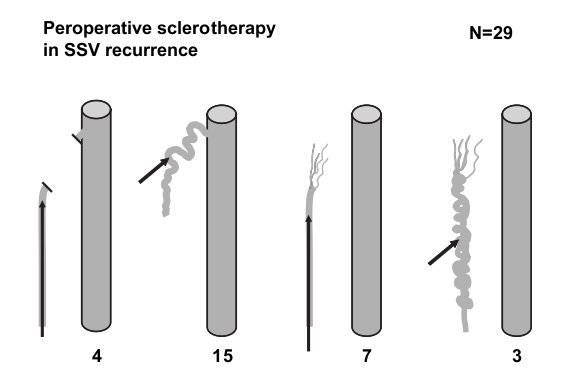
Table 1: CEAP classification of the patients treated by surgery and PFS
| CEAP « C » Class | N | % |
|---|---|---|
| C1 | 0 | 0.0 |
| C2 | 96 | 74.4 |
| C3 | 15 | 11.6 |
| C4a | 14 | 10.9 |
| C4b | 2 | 1.6 |
| C5 | 0 | 0.0 |
| C6 | 2 | 1.6 |
| Total | 129 | 100 |
Table 2: Classification of the patients treated by surgery and foam according to the CEAP clinical components
| Presence of | N | % |
|---|---|---|
| Teliangiectasias | 129 | 100 |
| Varicose veins | 129 | 100 |
| Venous oedema | 23 | 17.8 |
| Inflammation or pigmentation | 18 | 13.9 |
| Lipodermatosclerosis or white atrophy | 2 | 1.6 |
| Healed ulcer | 0 | 0.0 |
| Active ulcer | 2 | 1.6 |
Table 3: Classification of patients according to the REVAS system
| N | % | |
|---|---|---|
| Topographical | ||
| Groin | 52 | 40.3 |
| Thigh | 107 | 82.9 |
| Popliteal fossa | 43 | 33.3 |
| Lower leg | 122 | 94.6 |
| Source | ||
| No source | 12 | 0.9 |
| Pelvic abdominal | 28 | 22.2 |
| Sapheno-femoral junction | 48 | 37.2 |
| Thigh perforator | 29 | 22.4 |
| Sapheno-popliteal junction | 19 | 14.7 |
| Popliteal perforator | 6 | 4.6 |
| Gastrocnemiusl veins | 0 | 0 |
| Lower leg perforator | 1 | 0.8 |
| Saphenous trunk | ||
| Above knee great saphenous trunk | 68 | 52.7 |
| Below knee great saphenous trunk | 21 | 16.3 |
| Small saphenous vein | 11 | 8.5 |
| No | 43 | 33.3 |
| Date | 2007 |
| Auteurs | Eur J Vasc Endovasc Surg 2007;33:619-24 Denis Creton, EC A Pare, rue A Pare, 54100 F-Nancy, France, and 2Jean Francois Uhl, 113 Avenue Charles de Gaulle, 92 200 F-Neuilly sur Seine, France |






